Mylan (Viatris): Global Leaders in HIV Prevention
Stands as one of the world's most recognizable and trusted names in generic pharmaceuticals. With a massive global footprint serving more than 165 countries, they have been at the forefront of the fight against HIV/AIDS for decades.
FDA Approved Facilities
WHO Prequalified
Bioequivalent Formula
About Mylan (Now Viatris) Mylan, recently evolving into Viatris through its historic merger with Upjohn (a division of Pfizer), stands as one of the world's most recognizable and trusted names in generic pharmaceuticals. With a massive global footprint serving more than 165 countries, they have been at the forefront of the fight against HIV/AIDS for decades. This transition to Viatris is not just a name change; it represents the combination of Mylan's scientific agility with Upjohn's established commercial capabilities and legacy of iconic brands. This synergy ensures continued, stable access to critical medicines even in volatile markets. When you choose a Mylan product, you are choosing a medication manufactured to the strictest FDA and WHO standards for safety, efficacy, and bioequivalence. You are relying on a supply chain that is robust, transparent, and audited by the world's strictest health authorities.
Why Choose Mylan Products?
The Science of Bioequivalence: Mylan’s generics are not just "copies"; they are bioequivalent alternatives. This means they have been clinically proven to release the same amount of active ingredient into the bloodstream at the same rate as the brand-name drug (Truvada® or Descovy®).
Proven Track Record: Mylan’s antiretrovirals are used by millions of people worldwide every day. Their products are backed by decades of clinical data and real-world success in HIV prevention and treatment programs globally.
Certified Quality Assurance: Their manufacturing facilities meet rigorous international regulatory standards, undergoing regular, unannounced inspections by the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO) to guarantee purity, potency, and stability.
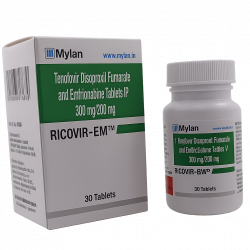
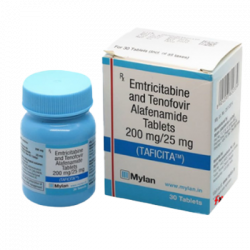
Innovation & Accessibility: Makers of widely trusted options like Ricovir-EM and the newer Taficita-EM, Mylan consistently innovates to provide affordable access to the latest prophylactic treatments, ensuring that financial status does not dictate health outcomes.
People also ask
Does Mylan (Viatris) have European regulatory approval?
Yes, Mylan operates state-of-the-art manufacturing sites that are regularly inspected and approved by the European Medicines Agency (EMA), ensuring that every batch meets the highest global standards for safety and purity.
Why is Mylan considered a global leader in ARV production?
Mylan has a decades-long legacy of pioneering affordable access to HIV treatment. They supply a vast portion of the world's antiretrovirals and are trusted by international health organizations for their consistent supply chain and rigorous quality control.
What is the difference between Mylan and Viatris?
There is no difference in product quality. Viatris is simply the new parent company formed by the merger of Mylan and Upjohn. You receive the same trusted medication from the same FDA-approved facilities.
Can I switch from Truvada to Mylan generic PrEP?
Absolutely. Most healthcare providers support switching to generics like Ricovir-EM to save on costs without compromising safety.
Truvada® and Descovy® are registered trademarks of Gilead Sciences, Inc. Mylan (Viatris) is not affiliated with Gilead Sciences. References to these trademarks are for comparative purposes only.